I hardly believed it myself, but all nine kids were here today!

We had a bit left to finish with our water experiment–graphing our results, so we quickly walked through the components of our experiment from beginning to end.

What we hadn’t taken time to write down before was our procedure.

And it’s not the trickiest part of the experiment, but it’s necessary. Just one more step in recording how we’ve done our experiment.

We talked more about our conclusions– Then it was time to graph our results.

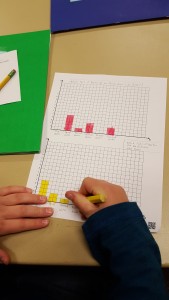
Last week we each had our water taste-test guesses and results on a piece of paper like this.

To graph our results, each of us had to consult our original guess and raise our hand for the water we THOUGHT we would like best.

Then we counted each raised hand, respectively, and graphed the class’s guesses as a whole.
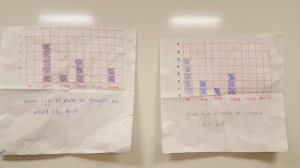
We did the same for the second graph, which showed us our actual water choices.
Here, it’s easy to see how many people are most satisfied by the drinking fountain down the hall.
All right. Putting those papers away…
Let’s talk about DENSITY!

To introduce density, I asked the kids to imagine that I was an amazing performer and they were all gathered at my concert.

If there were truly only nine of them at my concert and they were all spread out, how fast would I hit the ground if I dove off the stage?
Right. They figured I’d probably die.

But if they were all packed together really tightly in front of my stage, and I dove, what would happen?
- I’d still probably hit the ground. But…
- I wouldn’t hit it as hard, likely, or as fast, because their bodies would have cushioned mine.
So…what do we know about density?
The more dense something is, the more tightly packed together it is.
At this point, some were terrified that I might actually jump off the chair and crush them. Hoo boy.

So now we’re relating this to salt water and fresh water.

If salt water is more dense than fresh water, are items going to have an easier time sinking in salt water or floating in it?

To find out how much impact salty water has, we created our own.
We’ve got a bin of salt water.

And a bin of fresh water.

When our salt water is stirred, it looks a bit cloudy.

Awesome. Whose kid is that?!

Then we tested our items to see if they would sink or float in fresh water and sink or float in salt water.

The stuff that floated in the fresh water, we knew would float in the salt water because the salt water is more dense.

Some of the things that sunk in the fresh water…

Also sunk in the salt water. However, we noticed the items did not sink as quickly in the salt water.

Fun. Fun.
We’ll be getting our hands wet again next week!

I love these posts! I love seeing what they did and being able to discuss it later in the week! Keep it up!
LikeLike
Thanks, Nickie! We covered a lot of ground on Monday…so glad you’re getting a chance to talk about it at home!
LikeLike